Zinc Minerals:
Sphalerite, marmite, and to a lesser extent calamine are the only commercial significant minerals. These ores contain about 12% Zinc.
Minerals ores chemical formula
1. Zinc blend (or) Sphelerite ZnS
2. Marmalite (ZnFe)S
3. Calamine (or) Smithesonite ZnCO3
4. Heminorphite 4ZnO.ZnSiO2.3H2O
5. Hydrozincite 4ZnO.2CO2.3H2O
6. Willemite 2ZnO.SiO2
Selective Flotation of Zinc Sulphides:
Zinc is extracted pyrometallurgical as well as hydrometallurgically. Various pyrometallurgical techniques for the extraction of Zinc are
1. Distillation in horizontal retorts
2. Distillation in vertical retorts
3. Distillation in the electric furnace.
4. Zinc distillation in Blast Furnace (Imperial Smelting)
The blast furnace and hydrometallurgy processes are widely in practice. Out of these two, hydrometallurgy processes have some benefits
- Hydrometallurgical methods are ideally suited for lean and complex ores
- Hydrometallurgical operations ensure greater control than other conventional methods over energy steps in the processing of ores, resulting in the recovery of valuable by-products.
- Besides meeting the mounting demands for both the quantity and quality of metals, hydro-metallurgy can produce metals in a variety of physical forms such as powders, nodules and coherent surface deposits.
- Hydrometallurgical leaching operations are generally carried out at room temperature or at slightly elevated temperatures.
- In hydrometallurgy, the water liquor from the final recovery step can be recycled for the initial leaching operation.
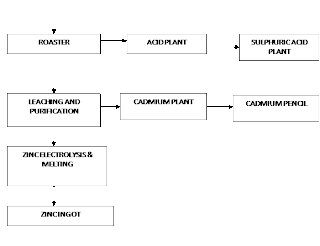
The hydrometallurgical route of the Roaster-Leach Electro-winning process is adopted for the extraction of electrolytic-grade Zinc.
Hydrometallurgical Process in Brief:
In Hydrometallurgical treatment, Zinc concentrates are roasted, then leached by a diluted Sulphuric Acid, Zinc passes into the solution according to the reaction
ZnO + H2SO4 —-> ZnSO4 + H2O
Silica and the bulk of Iron Oxides remain in the insoluble residue. Resultant ZnSO4 liquor is treated for removal of impurities and processed by electrolysis. Pure Zn settles of the cathode, O2 evolves at the anode, and H2SO4 that is accumulated in the solution is suitable for leaching roasted concentrate. The overall reaction being:
2ZnSO4 + 2H2O —-> 2Zn + 2H2SO4 + O2
Zinc deposited on the cathode is melted and cast into Ingots.
The Zinc concentrate is transported to the existing plant by rail. The received raw materials at the plant are stored in the storage yard for further use. The typical composition of the Zinc concentrate is given below which becomes feed to the process.
Component Percentage
Zinc 52 – 53
Sulfur 29 – 31
Iron 7 – 8
Lead 1.5 – 2.5
Copper 0.03 – 0.05
Cadmium 0.3
(GENERAL PROCESS FLOW DIAGRAM)
Related Topic
- NITRIC ACID Production Process
- Interphase Mass Transfer |mass transfer basics
- Manufacture Of 200 TPD Sulphuric Acid, Flow Sheet Of Production Process Plant
- ABSORPTION Operations And Equipment
- CURRENT TRANSFORMER TESTING METHODS |20 MVA POWER TRANSFORMER TESTING METHODS
- 20 MVA POWER TRANSFORMER TESTING METHODS | BUS BAR TESTING
- Hydrometallurgical Process And Smelting Operation
